

gondii parasites (IC 50 =2.0-3.5 μM) with considerable selectivities when taking their effects on non-malignant Vero cells into account. In addition, the new compounds 3 b, 3 c, and 4 a-c exhibited high activity against T. For instance, the new bromo- and iodo-substituted compounds 3 b/c and 4 b/c showed strong activity against L. Replacement of one of the aryl meta-methoxy groups by halogens such as chlorine, bromine and iodine led to distinctly increased antiparasitic activities.
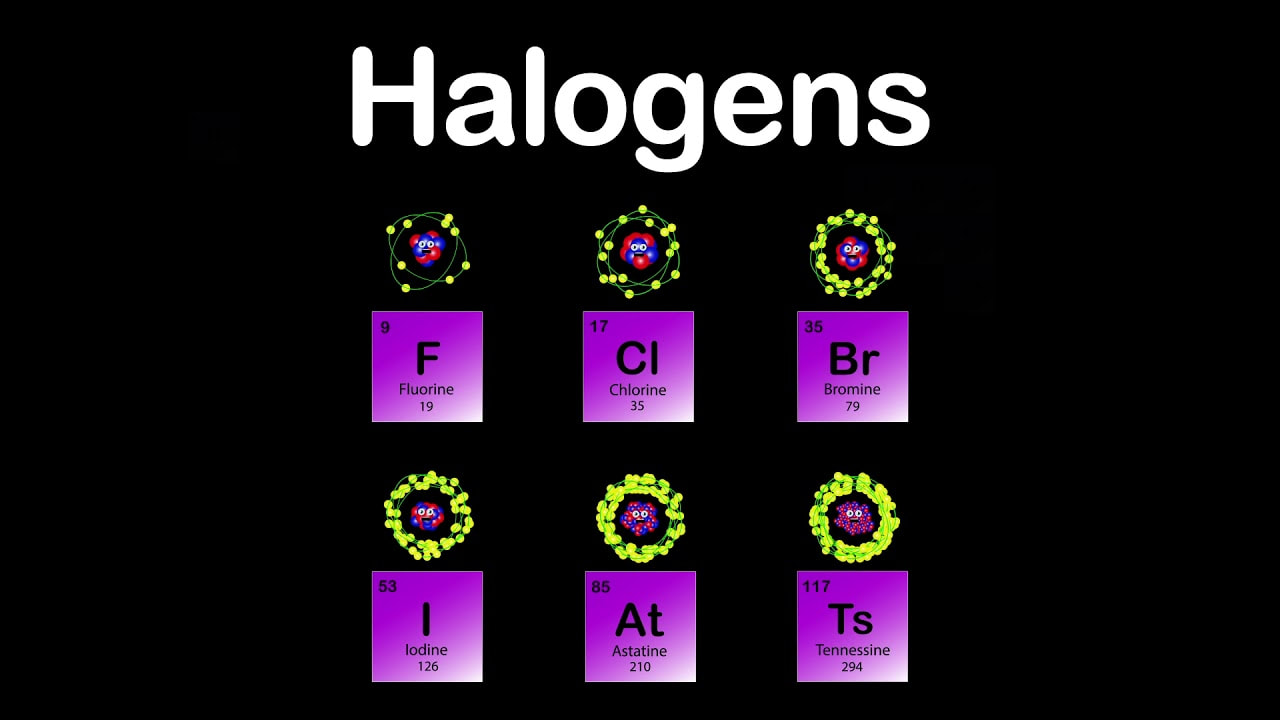
HALOGEN ACTIVITY SERIES SERIES
10 Organic Chemistry Laboratory, University Bayreuth, Universitätsstrasse 30, 95440, Bayreuth, Germany.Ī series of synthetic N-acylpyrrolidone and -piperidone derivatives of the natural alkaloid piperlongumine were prepared and tested for their activities against Leishmania major and Toxoplasma gondii parasites.9 Laboratory of Natural and Bio-active Substances, Faculty of Science, Tlemcen University, P.O.8 University Mohamed Khider, Department of Matter Sciences, BP 145 RP, Biskra, 07000, Algeria.7 Department of Chemistry, College of Science and Arts at Ar Rass, Qassim University, P.O.6 Laboratoire de Caractérisations, Applications et Modélisations des Matériaux, Faculté des Sciences de Tunis, Université Tunis El Manar, Tunis, Tunisia.5 Department of Genetics, University Bayreuth, Universitätsstrasse 30, 95440, Bayreuth, Germany.MolewaterplGD, Rotterdam (The, Netherlands.
4 Department of Medical Microbiology and Infectious Disease, Erasmus MC, University Medical Center Rotterdam, Dr.3 Department of Science Laboratories, College of Science and Arts, Qassim University, Ar Rass, 51921, Saudi Arabia.2 Department of Biology, College of Science and Arts, Qassim University, Unaizah, 51911, Saudi Arabia.1 Department of Clinical Nutrition, College of Applied Health Sciences, Qassim University, Ar Rass, 51921, Saudi Arabia.


 0 kommentar(er)
0 kommentar(er)
